
Any patient who presents with potential rabies or Australian bat lyssavirus exposure should be immediately referred to a doctor or medical centre.
Rabies is a nationally notifiable disease, so the Public Health Unit (PHU) must be contacted on 1300 066 055. You can contact the PHU at any time regarding a potential rabies or bat lyssavirus exposure, including after hours and on weekends. This includes returning travellers with overseas exposures. Exposure can occur through an animal scratch or bite that has broken the skin, or by direct contact of the virus with the mucosal surface of a person, sch as nose, eye or mouth.
Post-exposure prophylaxis
Post-exposure prophylaxis for rabies and other lyssaviruses includes prompt wound management, administration of post-exposure rabies vaccine and, in some cases, human rabies immunoglobulin.
The appropriate combination of these interventions and the number of vaccine doses depend on a risk assessment conducted by the PHU in conjunction with the treating doctor based on the patient’s exposure history.
The PHU will order the rabies vaccine and the immunoglobulin post-exposure treatment, which will be provided to the patient at no cost as a NSW-funded additional free vaccine.
Full rabies management advice is also available on South Eastern Sydney – HealthPathways and Sydney – HealthPathways.
Pre-exposure prophylaxis
Please encourage pre-exposure prophylaxis with rabies vaccine prior to travel to rabies endemic countries.
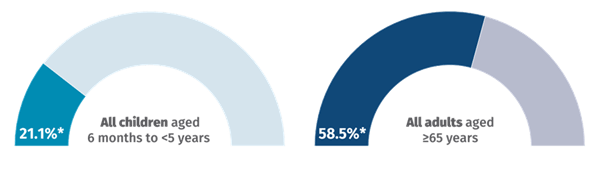
The National Centre for Immunisation Research and Surveillance (NCIRS) is monitoring and reporting influenza vaccination coverage data throughout the 2025 respiratory illness season.
The latest data, covering cumulative coverage percentages for the year up to 28 June 2025, are shown below.
Source: NCIRS Weekly Jab newsletter 4 July 2025. Subscribe here: ncirs.org.au/user/register
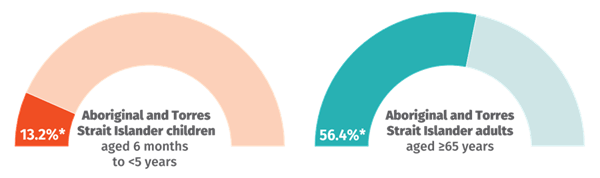
Register for a free influenza vaccination QI activity
CESPHN can support your practice in undertaking an influenza vaccination Quality Improvement (QI) activity . This QI program uses GoShare to send a personalised SMS to eligible patients who have not had a flu vaccine in 2025 to encourage them to book an appointment.
The flu vaccine recall program is only available to 20 practices in CESPHN, so be sure to register your interest promptly!
Contact immunisation@cesphn.com.au for more information
In response to reports about administration errors related to respiratory syncytial virus (RSV) prevention products, the Therapeutic Goods Administration (TGA) has published a safety update. The update is a reminder to prescribers and vaccination providers that each product is specifically indicated for different patient groups. It includes information on:
The RSV maternal vaccination kit is another valuable source of resources to help raise awareness about the risks of RSV in young children and the benefits of vaccination during pregnancy.
| REGISTER HERE 9 July 2025 18:00-19:00 AEST Online | Immunisation Coalition Webinar – 2025 RSV Update This webinar will provide an overview of RSV epidemiology, disease burden and an update on the new NIP listed maternal RSV vaccine and monoclonal antibody State funded programs from an indication and usage perspective. Consideration is also given to the vaccine for older adults. |
| REGISTER HERE 22 July 2025 19:00-20:00 AEST Online | Immunisation Coalition Webinar – Navigating newborn and infant RSV immunisation in general practice A multi-disciplinary panel of experts will discuss RSV immunisation in newborns and eligible infants in their second RSV season, and clarify use of nirsevimab under different seasonal and patient situations. |
| REGISTER HERE 23 July 2025 18:00-19:00 AEST Online | Immunisation Coalition Webinar – COVID-19 Update Webinar The Immunisation Coalition’s annual update on COVID-19 in Australia for GPs, Immunisation Nurses, Pharmacists, and other immunisation professionals will provide the latest facts about COVID-19 and vaccination. |