

RSV (Respiratory Syncytial Virus) is a virus that causes upper and lower respiratory tract infection and can cause severe disease, particularly in very young and older people.
Mother: Abrysvo vaccine is provided free on the NIP to pregnant women from 3 February 2025.
Infant: Beyfortus monoclonal antibody is provided free (NSW funded) to eligible infants.
The TGA has reported multiple instances of RSV administration errors including Arexvy administered to pregnant women (instead of Abrysvo), and Abrysvo administered to infants (instead of Beyfortus).
1 August 2025
ATAGI has released a statement on respiratory syncytial virus (RSV) immunisation products and prevention of administration errors. The statement follows recent reports to the TGA indicating that some RSV products have been used incorrectly.
The statement provides guidance on actions following inadvertent administration of the incorrect product, as well as clinical guidance. It should be read in conjunction with the RSV chapter of the Australian Immunisation Handbook and information on correct administration published by the TGA.
17 March 2025
NSW Health has received reports of Abrysvo® (RSV vaccine) incorrectly administered to children occurring in general practice. A NSW Health clinician alert was sent out to all GPs in NSW.
To help prevent errors, refer to the new RSV immunisation product reference guide developed to assist providers, available on the NSW RSV Prevention Program – Information for health professionals webpage under the resources section.
The introduction of new vaccine products can increase the risk of vaccine administration errors. Immunisation providers must ensure that a vaccine is clinically appropriate for the patient prior to administration. Some potential errors include:
Infant RSV immunisation
Eligible newborns will be offered Beyfortus™ in hospital under the NSW RSV Prevention Program. General practices can order Beyfortus™ where an eligible child has been missed in hospital or has not received 2nd RSV season dose in hospital. General practices are advised NOT to keep Beyfortus (nirsevimab) stock in their vaccine refrigerators.
Maternal RSV vaccine
Adult RSV vaccine
The RSV vaccine Abrysvo® is now free for eligible pregnant women under the National Immunisation Program (NIP) and is recommended between 28 to 36 weeks of pregnancy.
Getting Abrysvo® during pregnancy protects newborns via the transfer of maternal antibodies to the foetus. RSV vaccine in pregnancy reduces the risk of severe RSV disease in infants less than 6 months of age by about 70%.
Ordering: Abrysvo® vaccine can be ordered through the NSW State Vaccine Centre. *See shelf life extension*
Eligibility: Abrysvo® is only funded on the NIP for pregnant women. While Abrysvo® is registered for people aged 60 years and over, it is NOT funded on the NIP for this age group.
Recommendation: A single dose of Abrysvo® should be offered to all pregnant women between 28 to 36 weeks gestation. Abrysvo® can be given beyond 36 weeks gestation however infants may not be adequately protected if they are born within 2 weeks of the mother receiving the vaccine.
Timing: Abrysvo should be offered at the 28-week antenatal appointment to ensure a high level of antibodies are transferred to the baby before birth and maximise protection for babies who are born prematurely.
Co-administration: Abrysvo® can be co-administered with other antenatal vaccines including pertussis and influenza vaccines.
Reconstitution: Abrysvo® must be reconstituted using the vial adapter provided – refer to Abrysvo® instructions for use poster. Email austsupport@pfizer.com to order reconstitution poster for your clinic.
Administration: Abrysvo® is for intramuscular (IM) injection only, preferably in the deltoid region of the upper arm.
Documentation: Abrysvo® must be recorded on the Australian Immunisation Register (AIR), selecting the antenatal indicator to indicate a patient is pregnant at the time of vaccination. Vaccination should also be recorded on a patient’s ‘Yellow’ Antenatal Card (or other antenatal cards used in private settings). This card is used in hospital settings to determine a newborn infant’s eligibility for nirsevimab at birth based on the mother’s vaccination record.
Caution: Abrysvo® is the only RSV vaccine approved for use in pregnant women. Arexvy® vaccine should NOT be given to pregnant women.

Pfizer advises that the shelf life of the below listed batches of ABRYSVO® is extended from 24 to 36 months, following the Therapeutic Goods Administration (TGA) approval. The storage conditions remain unchanged and include the requirement to store ABRYSVO® in a refrigerator (2°C – 8°C) prior to reconstitution. Refer to the table below for the updated expiry dates.
| Batch number | Expiry date on package | Updated expiry date |
| LL2636 | 31.07.2025 | 31.07.2026 |
| LR6779 | 30.06.2025 | 30.06.2026 |
| LR6778 | 31.05.2026 | 31.05.2027 |
For ABRYSVO® stock with batch numbers different to those listed above, the expiry date remains unchanged.
Clinical information: The Immunisation Handbook has been updated and provides detailed information about the maternal Abyrsvo® vaccine.
Order RSV materials: Email austsupport@pfizer.com to order the following RSV materials developed to support the maternal RSV vaccination program: Abrysvo – Brochure for pregnant women, RSV awareness – Poster, RSV awareness – First Nations Poster, RSV – Consumer leaflet.

The RSV vaccine is available for eligible pregnant women under the NIP and is recommended between 28 to 36 weeks of pregnancy.
SKAI resources to support conversations
The Sharing Knowledge About Immunisation (SKAI) website has been updated with webpages to support conversations between providers and pregnant women about RSV vaccination in pregnancy.
These webpages include information about RSV disease and vaccine for pregnant women and their partners, and vaccination recommendations during the third trimester.
NSW Health Evidence Review
New maternal RSV program Evidence Review resource has been developed to support conversations about maternal RSV vaccination with Abrysvo between health professionals and pregnant women.
Review also the suite of resources on the RSV vaccination toolkit webpage and introduction to maternal RSV vaccine webpage.
Pfizer advises that the shelf life of the below listed batches of ABRYSVO® is extended from 24 to 36 months, following the Therapeutic Goods Administration (TGA) approval. The storage conditions remain unchanged and include the requirement to store ABRYSVO® in a refrigerator (2°C – 8°C) prior to reconstitution. Refer to the table below for the updated expiry dates.
| Batch number | Expiry date on package | Updated expiry date |
| LL2636 | 31.07.2025 | 31.07.2026 |
| LR6779 | 30.06.2025 | 30.06.2026 |
| LR6778 | 31.05.2026 | 31.05.2027 |
For ABRYSVO® stock with batch numbers different to those listed above, the expiry date remains unchanged.
Refer to the RSV Prevention Program – Information for health professionals for further information.
On 28 March, NSW Health sent a letter to GPs advising that the eligibility for the infant RSV immunisation has been expanded to include newborn infants born from 1 January 2025 (previously 17 March 2025) to receive nirsevimab. Vulnerable infants born 1 October 2024 to 31 December 2024 will continue to be offered nirsevimab if they meet the criteria for the NSW Vulnerable Babies Program. Refer to NSW RSV Prevention Program – Eligibility summary.
Ordering nirsevimab
GPs can order Beyfortus™ (nirsevimab) from the NSW State Vaccine Centre (SVC) ordering portal.
A maximum of 2 doses of nirsevimab 50mg can be ordered directly from the SVC portal as part of a monthly routine vaccine order.
GPs can order nirsevimab 100mg through the Nirsevimab Special Order Form on the SVC. Each order form can be used for up to 5 patients, and providers must ensure that the infant meets NSW eligibility criteria before placing order for nirsevimab 100mg.
All eligible infants are offered nirsevimab prior to discharge from hospital, so there is expected to be a limited number of eligible infants presenting to GPs.
Non-eligible patients cannot access nirsevimab through private prescription.
Clinical recommendations
Flow charts have been developed to assist providers to determine if an infant is clinically recommended to receive Beyfortus™ (nirsevimab). This does NOT necessarily mean they meet the eligibility criteria for NSW-funded Beyfortus™ (nirsevimab).
The NSW Health infant RSV prevention program will fund nirsevimab (Beyfortus™) for the following infants (including those without a Medicare card):
Eligibility resources:
Nirsevimab is a long-acting monoclonal antibody (mAB) for the prevention of RSV lower respiratory tract disease in neonates and infants born during or entering their first RSV season, and children up to 24 months of age who remain at risk of severe RSV disease through their second RSV season.
Presentation
Nirsevimab is available in two strengths: a 50mg 0.5mL prefilled syringe (purple) and a 100mg in 1mL prefilled syringe (blue).
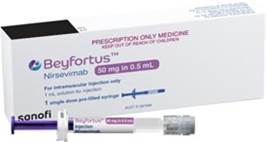 |
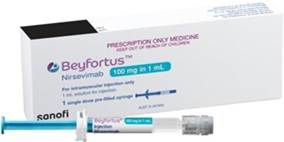 |
Dosage
The recommended dosage of nirsevimab depends on the infants body weight at time of dosing, and whether the infant is entering their first or second RSV season:
Injection site
Nirsevimab is administered intramuscularly (IM) at the injection site recommended by the Immunisation Handbook. The gluteal muscle should not be used routinely as an injection site because of the risk of damage to the sciatic nerve. If two injections are required, different injection sites should be used.
Recording multiple doses on AIR
Some children require administration of 2 x 100mg doses of nirsevimab, therefore two separate injections, which may occur in different limbs. However, AIR does not record multiple doses as it is assumed to be a reporting error/duplicate data. For this scenario, Services Australia recommends that vaccinations providers report this to the AIR as one single dose.
See the information sheet developed by Services Australia on how to report infant vaccinations to the AIR, including for infants without a Medicare card.
Co-administration
Nirsevimab can be given at the same time as routine childhood vaccines. When co-administered with vaccines, they should be given with separate syringes and at different injection sites. Refer to NCIRS injection site poster.
Administration errors
The NCIRS clinical guidance on RSV immunisation product administration errors webpage provides advice on the management of a range of possible RSV immunisation product administration errors. This includes situations where the incorrect product is administered and where a product is administered to a person who is younger or older than the approved age registered for that product.
The RSV Protection for Infants modules developed by Benchmarque Group provide participants with the skills and knowledge to work directly with families, parents and patients to increase understanding of RSV and the NIP. The modules are free to complete. These modules aim to give participants a baseline understanding of RSV information and an understanding of safe storage, preparation, and administration of Beyfortus® (nirsevimab), ensuring adherence to best practice
Using scenarios and communication frameworks, participants will learn how to engage with parents and caregivers, and use evidence-based insights to deliver proactive, informed care that minimises the impact of RSV and supports families in making informed immunisation decisions.
Order RSV materials: Email austsupport@pfizer.com to order the following RSV materials developed to support the maternal RSV vaccination program: Abrysvo – Brochure for pregnant women, RSV awareness – Poster, RSV awareness – First Nations Poster, RSV – Consumer leaflet.